The H5N1 vaccine, a critical tool in the fight against avian influenza, offers a comprehensive shield against this potentially deadly virus. This vaccine, meticulously engineered to combat the unique characteristics of H5N1, stands as a testament to scientific advancements and global health initiatives.
The H5N1 virus, a highly pathogenic strain of avian influenza, has raised global concerns due to its potential to cause severe respiratory illness in humans. With a deep dive into the virus’s genetic makeup, transmission mechanisms, and global distribution, we unravel the complexities of this formidable pathogen.
H5N1 Vaccine Overview
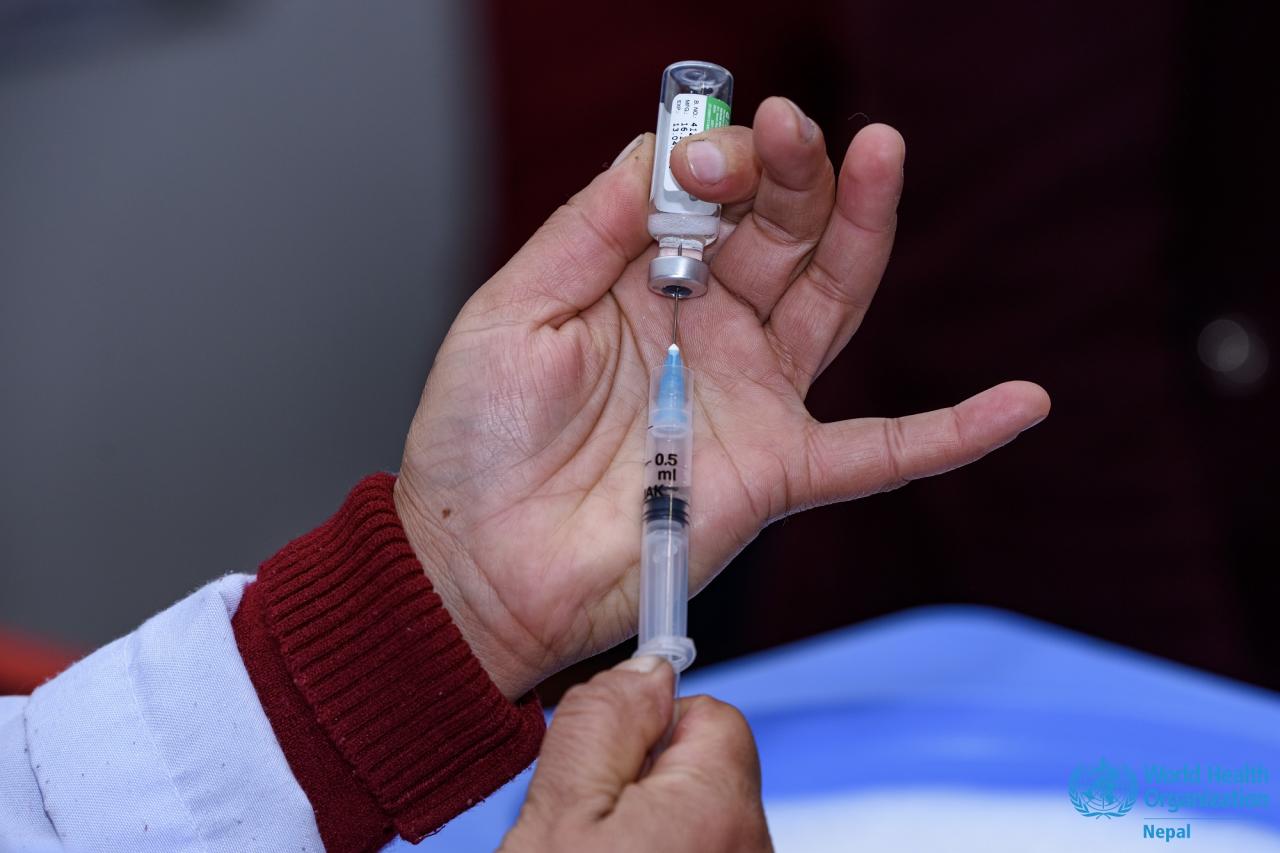
The H5N1 vaccine is designed to protect individuals from infection with the H5N1 influenza virus, a highly pathogenic strain that can cause severe respiratory illness in humans. The vaccine is composed of inactivated H5N1 virus particles, which stimulate the immune system to produce antibodies against the virus.
It is typically administered in two doses, several weeks apart.
The target population for the H5N1 vaccine includes individuals at high risk of exposure to the virus, such as poultry workers, healthcare professionals, and travelers to areas where the virus is known to be circulating. The vaccine has been shown to be safe and effective in preventing H5N1 infection in clinical trials.
H5N1 Virus Characteristics
The H5N1 virus is a subtype of the influenza A virus, which is characterized by its genetic makeup and virulence. The virus has a segmented genome, which allows for rapid genetic reassortment and the emergence of new strains. H5N1 is highly pathogenic in birds, and can also infect humans, causing severe respiratory illness.
Transmission of the H5N1 virus primarily occurs through contact with infected birds or their secretions. The virus can also be transmitted through contact with contaminated surfaces or objects. Human-to-human transmission of H5N1 is rare, but has been reported in cases of close contact with infected individuals.
The H5N1 virus has a global distribution, with outbreaks reported in Asia, Europe, Africa, and North America. The virus is particularly prevalent in poultry populations, and outbreaks in poultry can lead to significant economic losses.
H5N1 Vaccine Development
The development of the H5N1 vaccine began in the early 2000s, following the emergence of highly pathogenic H5N1 strains in Asia. Researchers worked to develop an inactivated vaccine that could safely and effectively protect individuals from infection.
Clinical trials of the H5N1 vaccine were conducted in multiple countries, and the vaccine was shown to be safe and immunogenic. The vaccine was approved for use in the United States in 2007, and has since been approved in several other countries.
The regulatory approval process for the H5N1 vaccine involved rigorous evaluation of the vaccine’s safety and efficacy. The vaccine was also subject to quality control measures to ensure its consistency and purity.
H5N1 Vaccine Distribution and Access
The distribution of the H5N1 vaccine is coordinated by public health organizations and governments. The vaccine is typically distributed to individuals at high risk of exposure to the virus, such as poultry workers, healthcare professionals, and travelers to areas where the virus is known to be circulating.
Factors influencing vaccine availability and accessibility include the global supply of the vaccine, the number of individuals at risk, and the resources available for vaccine distribution.
Public health organizations play a crucial role in ensuring equitable distribution of the H5N1 vaccine. They work to identify individuals at high risk, coordinate vaccine distribution, and monitor vaccine uptake.
H5N1 Vaccine Implementation
The H5N1 vaccine is administered through intramuscular injection. The vaccine is typically given in two doses, several weeks apart.
Vaccine hesitancy and resistance can impact the effectiveness of the H5N1 vaccine. Public health organizations work to address vaccine hesitancy by providing accurate information about the vaccine and its benefits.
The effectiveness of the H5N1 vaccine has been demonstrated in clinical trials. The vaccine has been shown to reduce the risk of H5N1 infection and severe illness. Herd immunity, achieved through high vaccination rates, can further reduce the risk of H5N1 transmission.
H5N1 Vaccine Research and Future Directions
Ongoing research on the H5N1 vaccine is focused on improving vaccine efficacy and developing new vaccine technologies. Researchers are working to develop vaccines that can provide broader protection against different strains of the H5N1 virus.
New vaccine technologies, such as mRNA vaccines and viral vector vaccines, are being explored for their potential to improve vaccine effectiveness and reduce side effects.
The development of universal influenza vaccines is a long-term goal of influenza research. Universal vaccines would provide protection against a wide range of influenza viruses, including H5N1, and could significantly reduce the burden of influenza.
Ending Remarks

As we delve into the intricate world of H5N1 vaccine development, we witness the tireless efforts of researchers and scientists. From the initial research and clinical trials to the stringent regulatory approval process, we uncover the challenges and breakthroughs that have shaped the vaccine’s journey.
The effective distribution and accessibility of the H5N1 vaccine remain paramount. We explore the strategies employed to ensure equitable access, examining the role of public health organizations and governments in safeguarding communities worldwide.
The successful implementation of the H5N1 vaccine hinges on proper administration and monitoring protocols. We delve into the potential impact of vaccine hesitancy and resistance, emphasizing the importance of evidence-based decision-making and community engagement.
With an eye towards the future, we explore ongoing research on H5N1 vaccine improvement and the development of new vaccine technologies. The quest for universal influenza vaccines holds immense promise, offering the potential to revolutionize our approach to influenza prevention.